Explain Different Type of Hydrogen Bond With Example
Get help with your Chemical bond homework. For example the covalent bond present within a hydrogen chloride HCl molecule is much stronger than any bonds it may form with neighboring molecules.
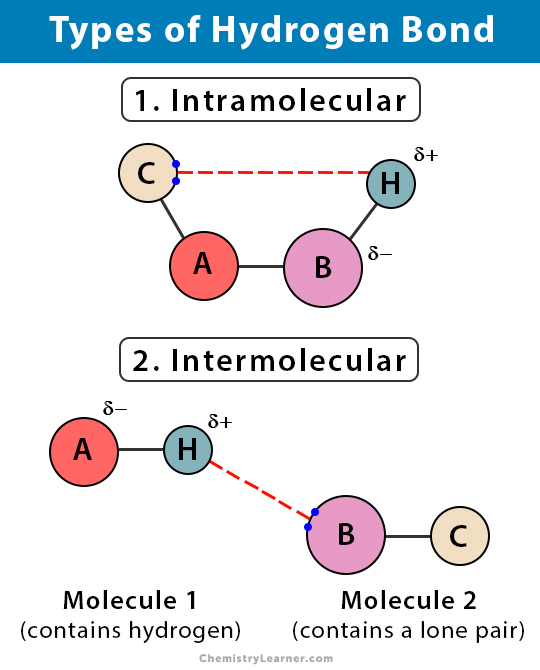
Hydrogen Bond Definition Types And Examples
Access the answers to hundreds of Chemical bond questions that are.

. Types of Attractive Intermolecular Forces. For example in the hydrogen-bonded systems below the acetic acid dimer the top hydrogen bond increases both the acidity of the hydrogen and the basicity of the oxygen in the bottom hydrogen bond. For example the covalent bond present within a hydrogen chloride HCl molecule is much stronger than any bonds it may form with neighboring molecules.
The strength of the bond between each atom is equal. For example you could have carbon-14 and nitrogen-14. 102 Reactions of Alkenes.
Addition of Hydrogen Halide to Alkenes Alkenes undergo a large variety of reactions. This bond is also much stronger compared to the normal hydrogen bond. Types of Attractive Intermolecular Forces.
Draw a plausible mechanism for this side reaction of GAPDH. The polarities of the carbonhalogen bond are in the same order. The double bond is the reactivity center of alkene this is mainly because.
Electrostatic interaction involving a. For example an isotope with 6 protons and 6 neutrons is carbon-12 or C-12. Moreover only a single measurement of product concentrations from a single sample is required.
An isotope with 6 protons and 7 neutrons is carbon-13 or C-16. Before we give you the nucleotide definition here are some helpful definitions of words well use when discussing nucleotides. Cooperativity of hydrogen bonding is observed in base pairing and in folded proteins.
This experiment type ensures that both C-H and C-D bond functionalizations occur under exactly the same conditions and the ratio of products from C-H and C-D bond functionalizations can be measured with much greater precision than the rate constants in Experiment A. Each hydrogen bond makes the other stronger than it would be in isolation. Well go over the nucleotide definition the different types of nucleotides out there what makes each type of nucleotide unique and why nucleotides are involved in nearly all cellular activities.
In chemistry orbital hybridisation or hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals with different energies shapes etc than the component atomic orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theoryFor example in a carbon atom which forms four single bonds the valence-shell s orbital combines. Strength of the hydrogen bond is determined by the coulombic interaction between the lone-pair electrons of the electronegative atom of one molecule and the hydrogen atom of other molecule. Therefore hydrogen bonds are powerful force in determining the structure and properties of many compounds for example proteins and nucleic acids.
This is a special type of hydrogen bond where the proton is usually placed in the middle between two identical atoms. Chemical Bond Questions and Answers. Electrostatic interactions of permanent dipoles in molecules.
Note the mass number of two isotopes may be the same even though they are different elements. The symmetric hydrogen bond is a type of a three-centre four-electron bond. At first glance these reactions appear to be quite different however detailed studies indicated that the different mechanism all share some common features.
Electrostatic interactions of permanent dipoles in molecules. For example glyceraldehyde- 3-phosphate dehydrogenase GAPDH which normally catalyzes the oxidative phosphorylation of glyceraldehyde-3-phosphate can slowly convert erythrose-4-phosphate an intermediate in the pentose phosphate pathway to 14-bisphosphoerythronate. For example the bond dissociation energies of carbonhalogen bonds increase in the order CI CBr CCl CF.
When a carbonhalogen bond breaks so that one electron remains with each fragment a process called homolytic bond cleavage the electropositive element carbon must recover its electron from.
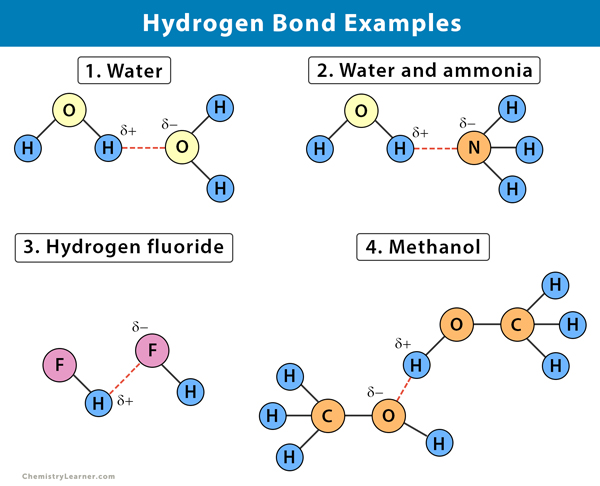
Hydrogen Bond Definition Types And Examples
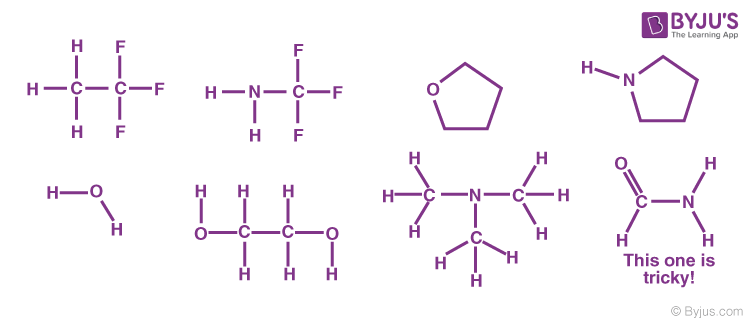
Hydrogen Bonding Properties Effects Types Examples Of Hydrogen Bond
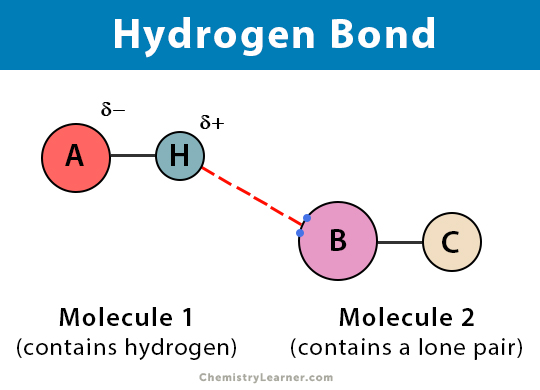
Hydrogen Bond Definition Types And Examples

What Are Types Of Hydrogen Bonds Explain With Suitable Examples Brainly In
0 Response to "Explain Different Type of Hydrogen Bond With Example"
Post a Comment